Clinical Trial
Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices
Artificial Intelligence (AI) has long promised to increase healthcare affordability, quality, and accessibility but FDA, until recently, had never authorized an autonomous AI diagnostic system…
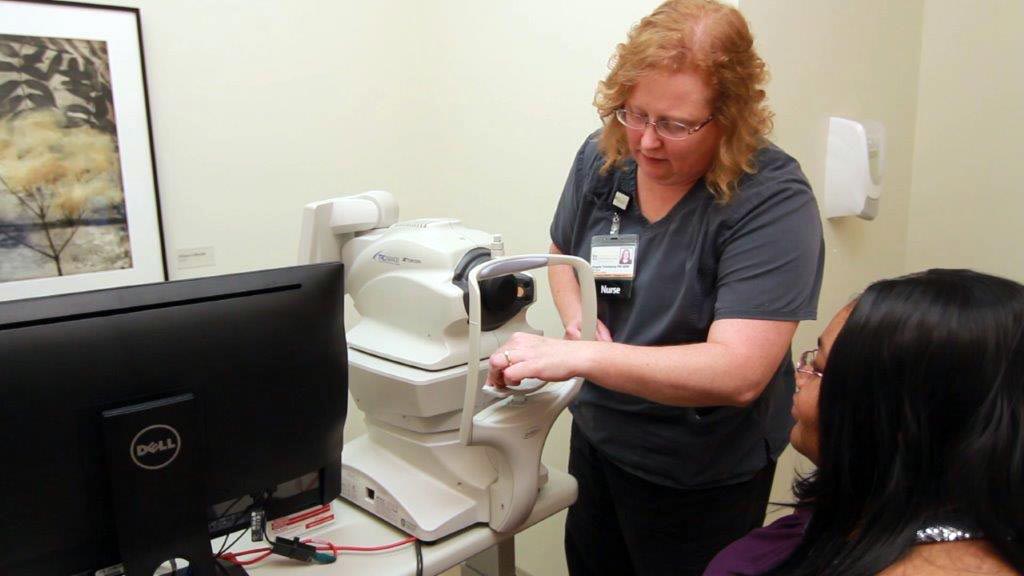
Introduction
People with diabetes fear visual loss and blindness more than any other complication. Diabetic retinopathy (DR) is the primary cause of blindness and visual loss among working-age men and women in the United States and causes more than 24,000 people to lose vision each year. Adherence to regular eye examinations is necessary to diagnose DR at an early stage when it can be treated with the best prognosis and have resulted in substantial reductions in visual loss and blindness.
Results
-
900: Enrolled in study
-
892: Intention to screen (ITS)
-
819: Analyzable: available for statistical analysis
-
621: Reading Center mtmDR negative
-
198: Reading Center mtmDR positive
Download Full Publication
Fill out this form to download the LumineticsCore® (formerly known as IDx-DR) Pivotal Trial Publication