LumineticsCore® - Overview | Play Video

LumineticsCore® is an Al diagnostic system that autonomously diagnoses diabetic retinopathy in people living with diabetes.
-
Diagnostic results at the point-of-care
-
Reimbursable with CPT Code 92229
-
Closes HEDIS care gap
-
A simple user interface
-
Customized workflow integration solutions
-
Algorithm diagnoses within 30 seconds of image submission
-
No need for specialist overread or telemedicine call backs
How LumineticsCore® Works
1
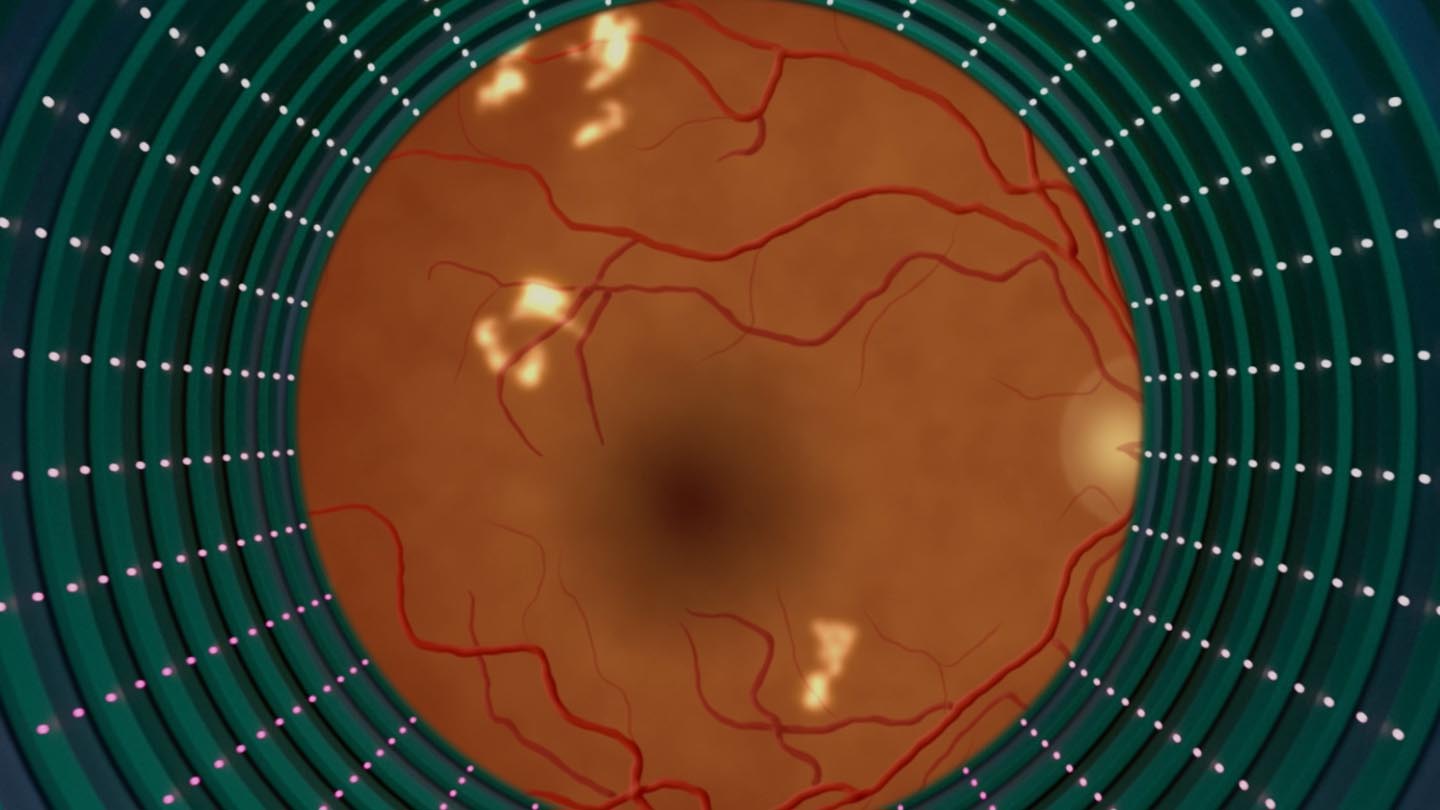
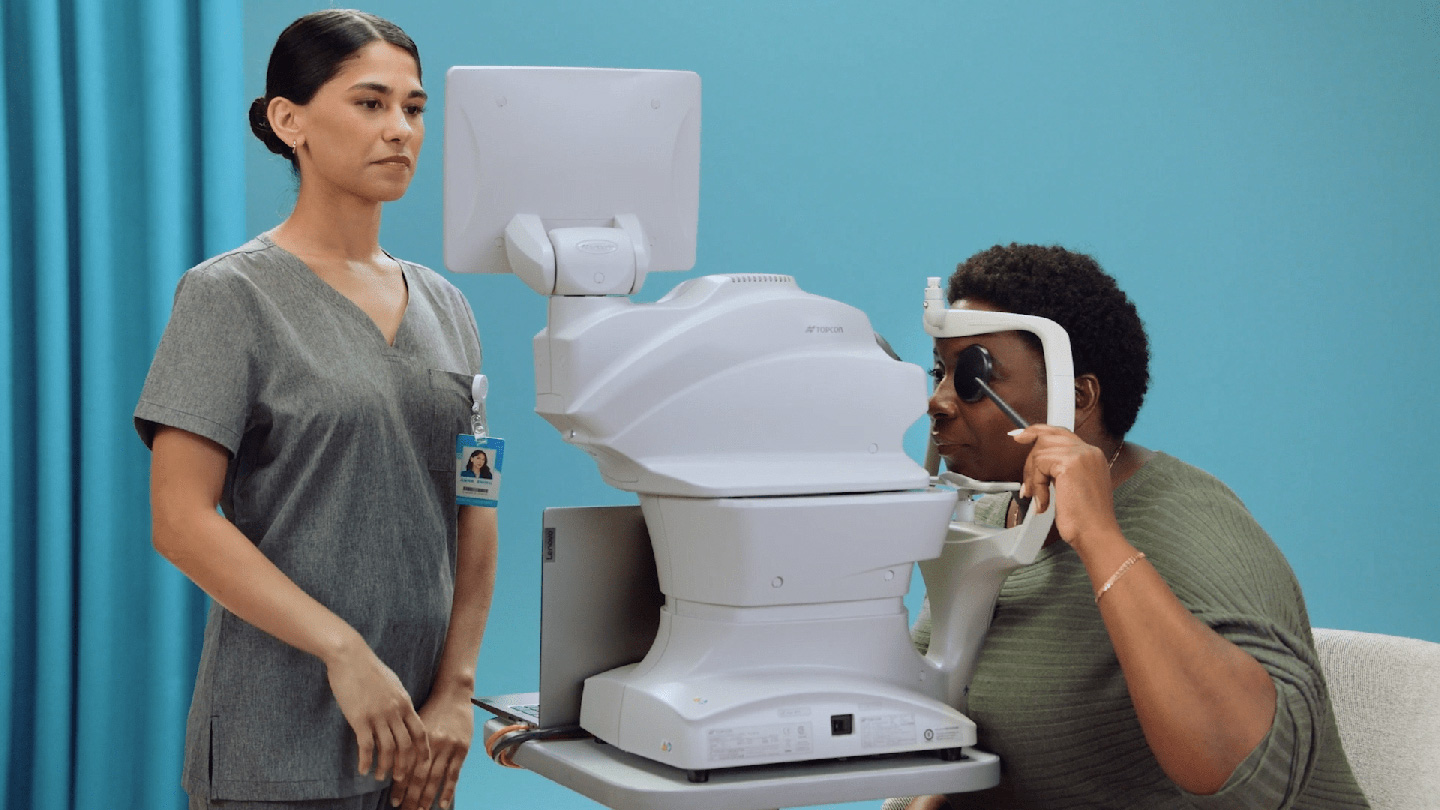
Using a fundus camera, the trained operator captures two images per eye
2
The images are submitted to LumineticsCore®



3
LumineticsCore® analyzes images for signs of diabetic retinopathy, providing results in less than a minute
Results
No diabetic retinopathy detected: Retest in 12 Months
Results
Diabetic retinopathy detected: Refer to an eye care professional
Our Approach to AI Driven Diagnostics
AI the Right Way®
LumineticsCore® is modeled to look for and make decisions based on the same clinical disease detectors that eye care specialists would use.
-
Diagnostic AI built on an ethical foundation
-
Diagnostic results at the point-of-care
-
Infinitely scalable
High Performance in a
Real-World Clinical Setting
Accessibility
LumineticsCore® has been trained and validated to mitigate bias and ensure the system works equally well for all people, regardless of sex, race, or ethnicity. For more information, read the LumineticsCore® pivotal trial.

Who is LumineticsCore® for?
Digital Diagnostics is impacting patient care across the globe.
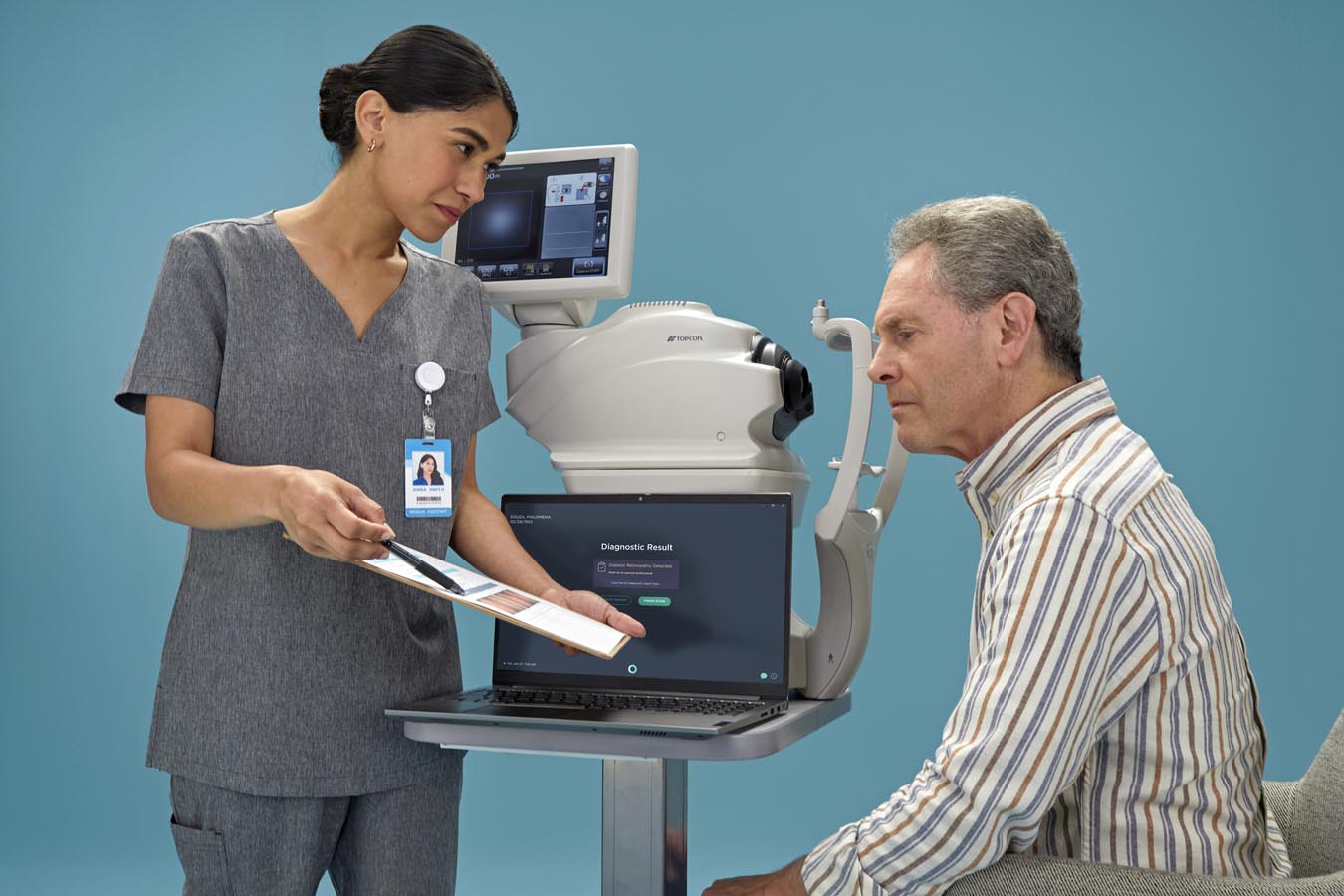
Front-Line Care Providers
LumineticsCore® is for medical professionals who want to leverage AI innovation to provide patients with a rigorously validated and ethically designed system for diabetic retinopathy diagnosis. LumineticsCore® results are provided at the point-of-care, allowing providers to present actionable next steps during the initial patient encounter. LumineticsCore® also provides the opportunity for improve quality measure performance for the eye exam for diabetes and can be reimbursed with CPT® code 92229.
Ophthalmologists & Optometrists
LumineticsCore® is for eye care providers to help address a known gap in care and improve patient access to the eye exam for diabetes. LumineticsCore® results can help increase actionable referrals by identifying patients with diabetic retinopathy who are not already under the care of an eye care provider. This can help increase access for the patients who are most likely to need timely vision-saving interventions.

Payor Community
LumineticsCore® is for payors to improve healthcare efficiency and access to care for enrollees while supporting the Patient Centered Medical Home (PCMH).
-
Cost savings over the life-time of care
-
Prevent blindness through early disease detection
-
100% compliance for diabetic retinopathy exams saves > $5B/year
Patients
LumineticsCore® is for adults (22 years of age or older) diagnosed with diabetes who have not been previously diagnosed with diabetic retinopathy and are not currently under the care of an eye care specialist. LumineticsCore® is installed at point-of-care locations to increase patient access and convenience.

Indications for Use
LumineticsCore® is intended for use by health care providers to automatically detect more than mild diabetic retinopathy (mtmDR) in adults (22 years of age or older) diagnosed with diabetes who have not been previously diagnosed with diabetic retinopathy. LumineticsCore® is indicated for use with the Topcon NW400.
LumineticsCore® FAQ
What is LumineticsCore®?
LumineticsCore® detects more than mild diabetic retinopathy, which is ETDRS level 35 or higher at the point-of-care to provide the patient with a diagnostic report without a physician evaluation. After completing a rigorous prospective, preregistered clinical trial at primary care sites across the country, LumineticsCore® became the first FDA De Novo-cleared AI diagnostic system to make a diagnosis without physician input. LumineticsCore® is deployed in point-of-care offices across the country.
What are the indications for LumineticsCore®?
LumineticsCore® is intended for use by health care providers to automatically detect more than mild diabetic retinopathy, which is ETDRS level 35 or higher in adults (22 years of age or older) diagnosed with diabetes who have not been previously diagnosed with diabetic retinopathy.
Warnings
- LumineticsCore® does not replace a full eye exam, but it will find diabetic retinopathy that needs to be examined by an eye doctor. Patients who have blurry vision or other symptoms of vision loss should see an eye doctor right away.
- While LumineticsCore® is reliable, it is not perfect. It is possible that LumineticsCore® is unable to detect disease that is present, or possibly give a false positive diagnosis.
To review the LumineticsCore® Indications for Use click here.
How does LumineticsCore® work?
Using a fundus camera, an operator captures high-quality images of the patients’ eyes. The images are then submitted to LumineticsCore® where they are analyzed for signs of diabetic retinopathy. A diagnostic result is provided during the patient encounter with follow up instructions for the patient based on the diagnosis. If actionable disease is detected, the patient is referred to an eye care professional, which has been shown to increase the chances of the patient following through with the referral appointment. If no actionable disease is detected the patient is advised to get retested in 12 months based on AAO annual exam guidelines.
Who is LumineticsCore® for?
Patients
LumineticsCore® is for adults (22 years of age or older) diagnosed with diabetes who have not been previously diagnosed with diabetic retinopathy and are not currently under the care of an eye care specialist. LumineticsCore® is installed at point-of-care locations to increase patient access and convenience.
Providers
LumineticsCore® is for medical professionals who want to provide patients with rigorously validated and ethically designed diagnostic results at the point-of-care, allowing for more comprehensive diabetes care and improved performance with eye exams for people with diabetes quality measures.
Payors
LumineticsCore® is for payors to help improve healthcare efficiency and access to care for enrollees while supporting the Patient Centered Medical Home (PCMH).
Eye Care Providers
LumineticsCore® is for eye care providers to help address disparities and improve access to care as part of the eye exam for diabetes delivery model. LumineticsCore® results can lead to more actionable referrals, identifying disease in patients not already under the care of an eye care provider, who are most likely to need timely vision saving interventions.